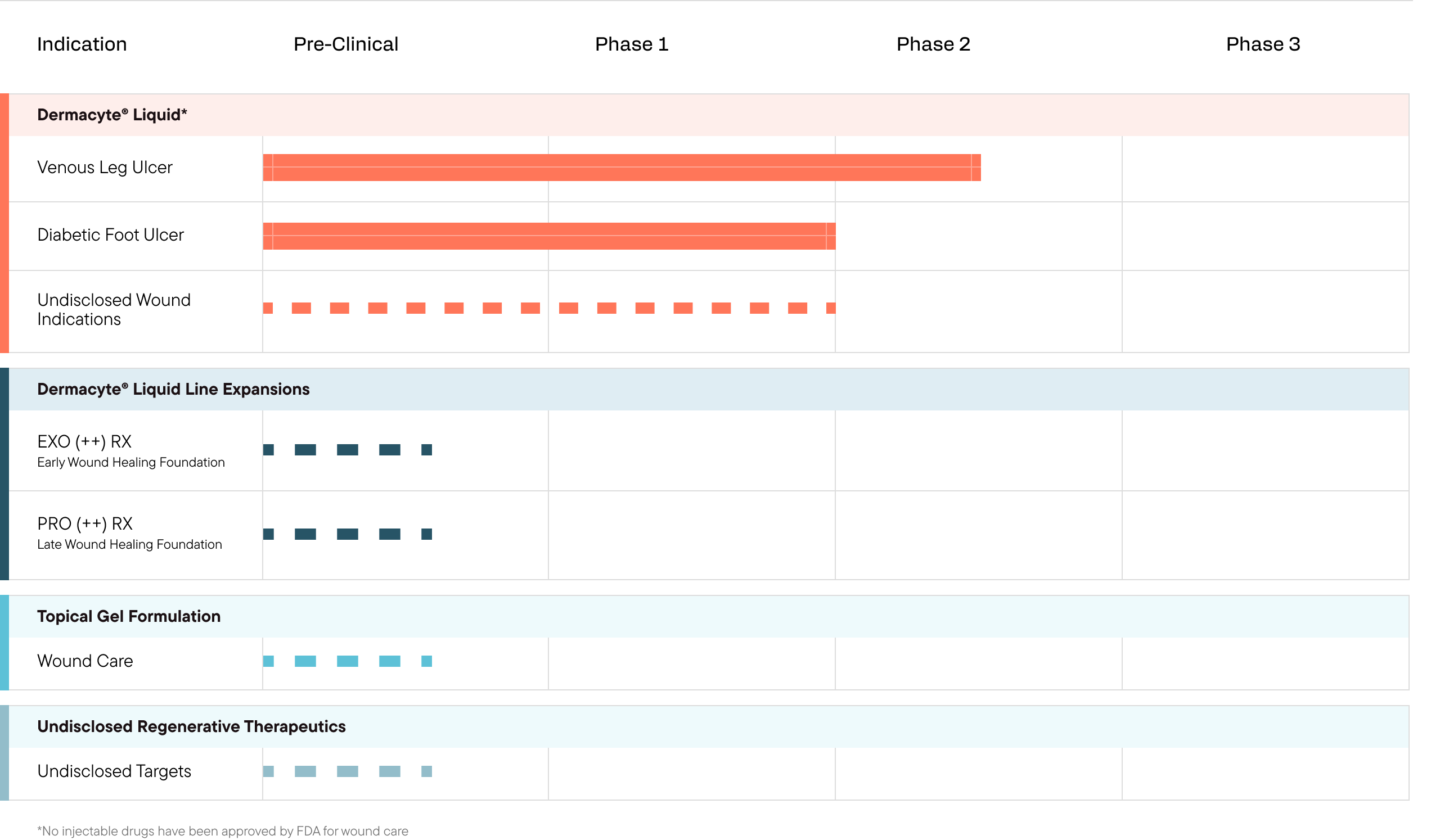
Pipeline Fueled by Bold Science
Merakris is developing a pipeline of first-in-class regenerative therapy candidates designed to combat a broad range of diseases with high unmet clinical needs. Our immediate focus is on the therapeutic area of complex wound healing including venous leg ulcers (VLUs) and diabetic foot ulcers (DFUs).
Our Pipeline Overview
Clinical Trial for Venous Leg Ulcer
VLUs account for
60-80%
of all venous disease linked leg ulcerations
Prevalence of VLUs
1.1M
Number of Americans with VLUs
60%
of VLUs progress to chronic wounds
*Probst, S., Weller, C.D., Bobbink, P. et al. Prevalence and incidence of venous leg ulcers—a protocol for a systematic review. Syst Rev 10, 148 (2021). https://doi.org/10.1186/s13643-021-01697-3
The high prevalence and severe complications of VLUs underscore the immediate need for more effective therapeutic solutions.
Merakris’ Dermacyte Amniotic Wound Care Liquid is currently being studied in a Phase II clinical trial for the treatment of non-healing venous leg ulcers.
Another Area of Focus
Another area of immediate focus at Merakris Therapeutics is in the treatment of non-healing chronic Diabetic Foot Ulcers (DFU).
Diabetics have
19-34%
lifetime risk of developing DFU
DFU patients have
20%
lifetime incidence of lower-extremity amputation
5-year DFU mortality rate
50-70%
McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care. 2023 Jan 1;46(1):209-221. doi: 10.2337/dci22-0043. PMID: 36548709; PMCID: PMC9797649.