Chronic wounds are a growing public health concern
Chronic, non-healing wounds impact millions of patients and represent a significant and growing burden on public health. In a study by Armstrong D.G. et al. (2020), the mortality and costs of care for foot complications in diabetic patients were found to be comparable to those of cancer, highlighting the severity of this issue.
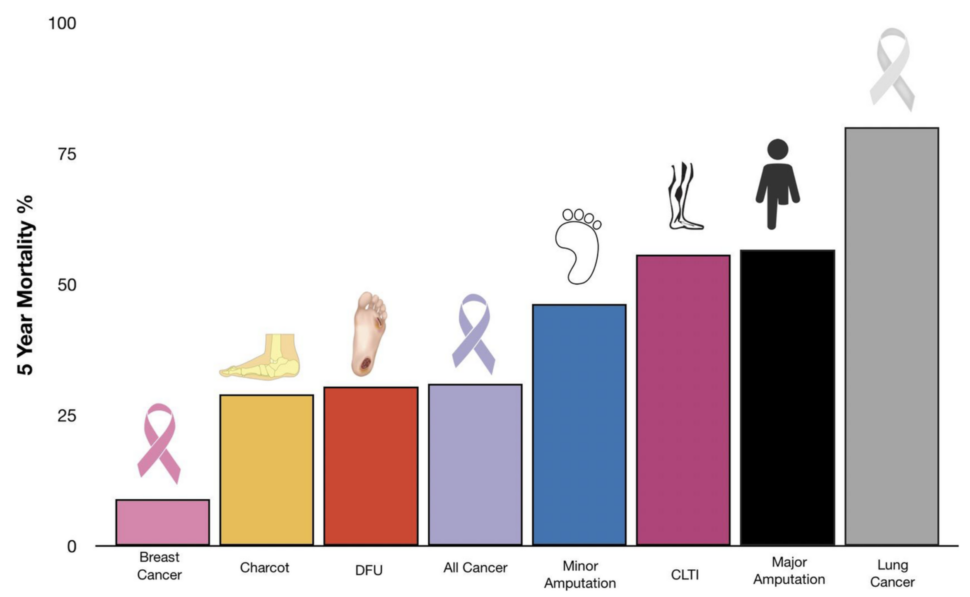
Armstrong, D.G., Swerdlow, M.A., Armstrong, A.A. et al. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res 13, 16 (2020)
Standard wound care methods often fall short for many patients, leaving them to suffer pain, infections, and reduced quality of life when wounds fail to close.
A new biologic therapy from Merakris Therapeutics could completely revolutionize care for those with hard-to-heal chronic wounds.
Traditional wound management and its limitations
Typical wound management approaches include gauze, foam dressings, films, grafting, vacuum devices, and more. However, these passive methods have significant limitations:
Gauzes
- Requires frequent changing (increases overall cost)
- Not effective for moist wounds
Foams
- Does not work on draining wounds or those with eschar
- Requires daily changing
Thin Film Dressings
- Does not absorb fluid
- Unsuitable for draining wounds
Shock Wave Therapy
- Can cause pain and hematoma at wound site
Negative Pressure Wound Therapy
- Challenging to maintain specific vacuum
Skin Grafting
- Time intensive
- Prone to infection
These methods lack robust healing mechanisms to activate the body’s natural regeneration processes. For difficult chronic wounds, more active solutions are needed.
Advanced biologic paradigm in wound care
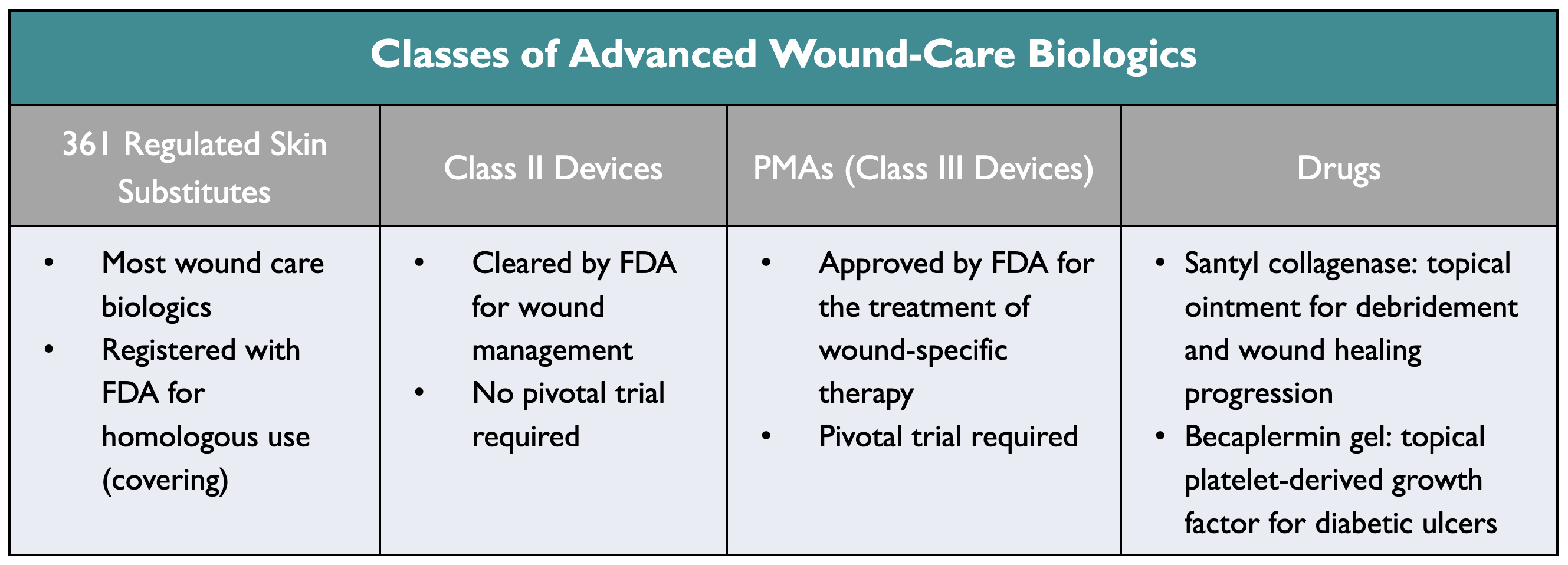
Most wound-care biologics are cleared based on homologous use as a covering or as a device for skin growth, with limited validated biologic wound-healing effects. These include 361 Regulated Skin Substitutes, Class II Devices, and PMAs (Class III Devices) Drugs, such as Santyl collagenase and Becaplermin gel.
Dermacyte Amniotic Wound Care Liquid*: A revolutionary approach to wound healing
Dermacyte Liquid represents a first-in-class biological drug for wound healing, currently in active Phase II clinical trials to support both early and late wound healing processes. This injectable drug offers an alternative to topical products, removing the hassle of moving and the associated shear forces on grafts.
How Dermacyte Amniotic Wound Care Liquid* Works
Dermacyte contains proteins and extracellular vesicles (EVs) demonstrated to be necessary for cell migration, stimulate myofibroblast activation (MFA), induce epithelial to mesenchymal transition (EMT), and resolve mesenchymal to epithelial transition MET.
In-Vitro Efficacy of Dermacyte Amniotic Wound Care Liquid*
In-vitro scratch wound closure models show that Dermacyte’s exosomes are key to promoting wound closure. The model demonstrates closure initiation in exosome-containing models, with limited closure in exosome-depleted amniotic fluid models.
Clinical Trials for Dermacyte Amniotic Wound Care Liquid*
Dermacyte Liquid is being studied in a 2-part study for the treatment of non-healing venous stasis ulcers (VSU). The target indication is for use in treating non-infected VSU that fail to show improvement after a minimum of 4 weeks of standard wound therapy. The primary objective is to evaluate the safety and tolerability of Dermacyte Liquid for the treatment of a lower limb extremity VSU. More details of the trial can be read here
Merakris Therapeutics’ focused research has led to a significant advancement in wound care that could potentially change the future landscape of chronic wound treatments. This development promises more effective and efficient healing solutions, bringing new hope to patients.
*Dermacyte® Liquid is an investigational new drug and has not been approved for marketing by the US FDA